How to measure respiration rates in Drosophila
How to measure respiration rates in Drosophila
Thu, Jun 13 2024

In this article, we showcase how the microplate respirometry system provides an easy to use, high throughput respirometry tool for determining oxygen consumption rates in various life stages of terrestrial insects, here using fruit flies (Drosophila melanogaster and Drosophila littoralis). The project was performed at Aarhus University in Denmark in collaboration with Prof Jesper G. Sorensen at Aarhus University in Denmark.
The first part of the project looked at the respiration rates in eggs, third instar larvae, and pupae of D. melanogaster. The fruit flies were maintained at 19 °C, and were in the following life stages:
- melanogaster eggs: 0-12 hours old
- melanogaster larvae: 6-7 days since egg stage
- melanogaster pupae: 8-9 days since egg stage
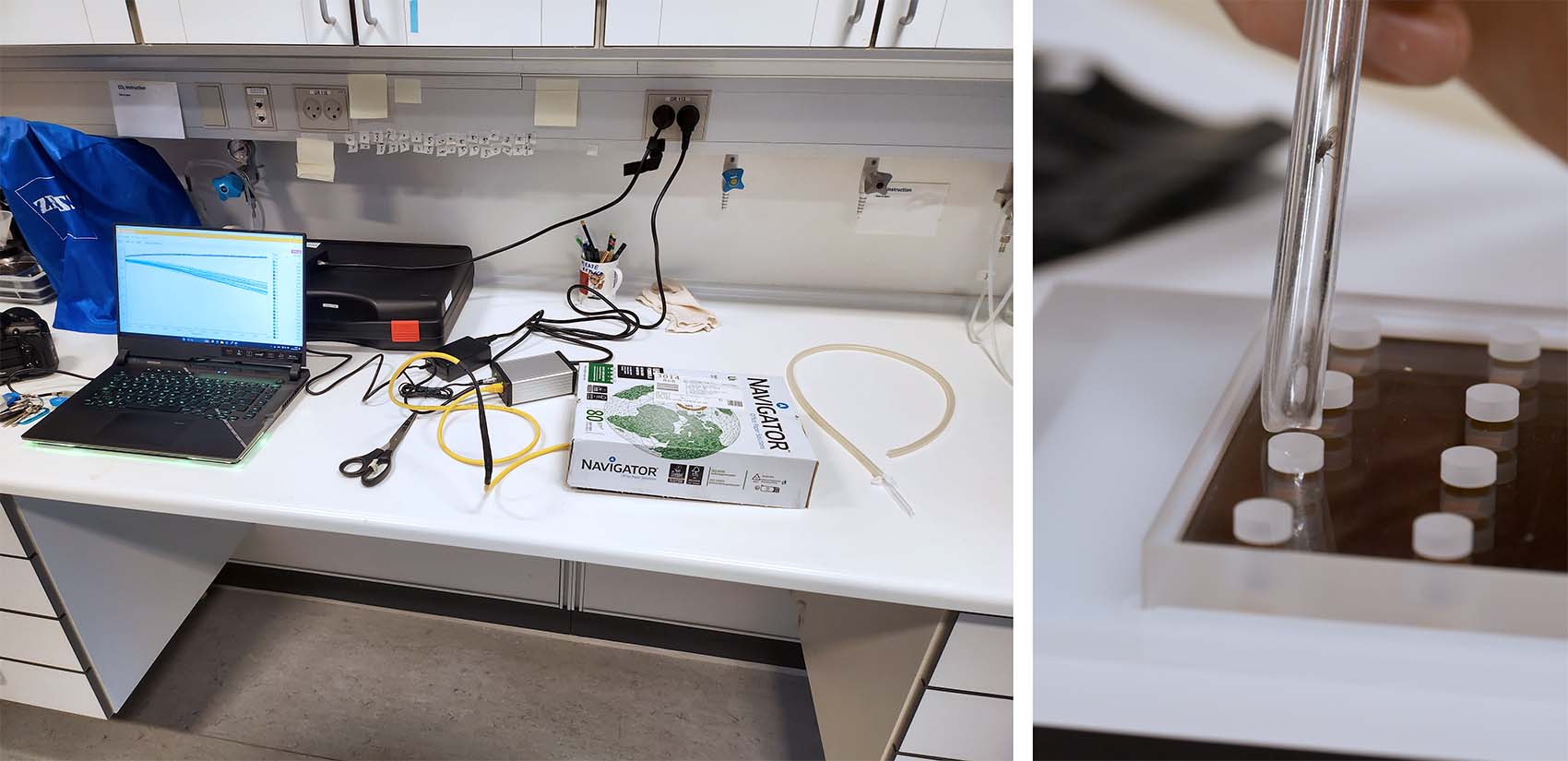
The first trial had 10 x eggs + 10 x pupae (+ 4 x blanks). The eggs and pupae were transferred directly from their food solution/colony and placed individually in a well in the 80 µl glass microplate. The wells were then closed using gas-tight non-toxic sealing film, and placed onto the microplate reader. The microplate reader and glass plate were then covered with an opaque plastic box, to prevent ambient light from interfering with the optical oxygen sensor inside each well, and left at room temperature (21 °C). Here is a picture of the setup:

The MicroResp™ v1.1 software was used to calibrate the oxygen sensors, set up the plate and experiment settings, and to log data. Here are the exported data graphs:
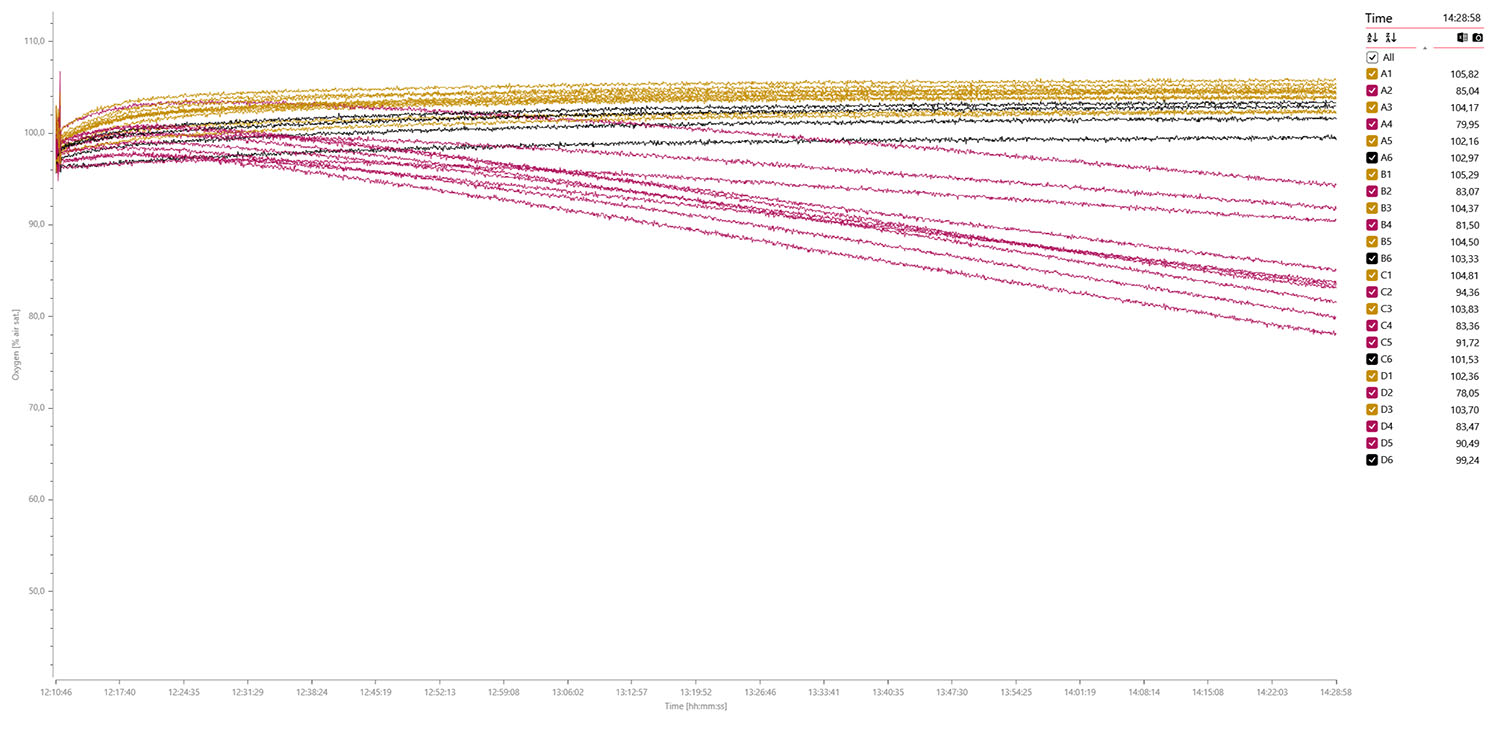
Oxygen data graph for 10 x eggs (dark golden), 10 x pupae (purple), and 4 x blanks (black) measured over 2 hours and 18 min.
The next trial used 20 x third instar larvae (dark cyan) and 4 x blanks (black), and data was logged for 1 hour and 35 min.:
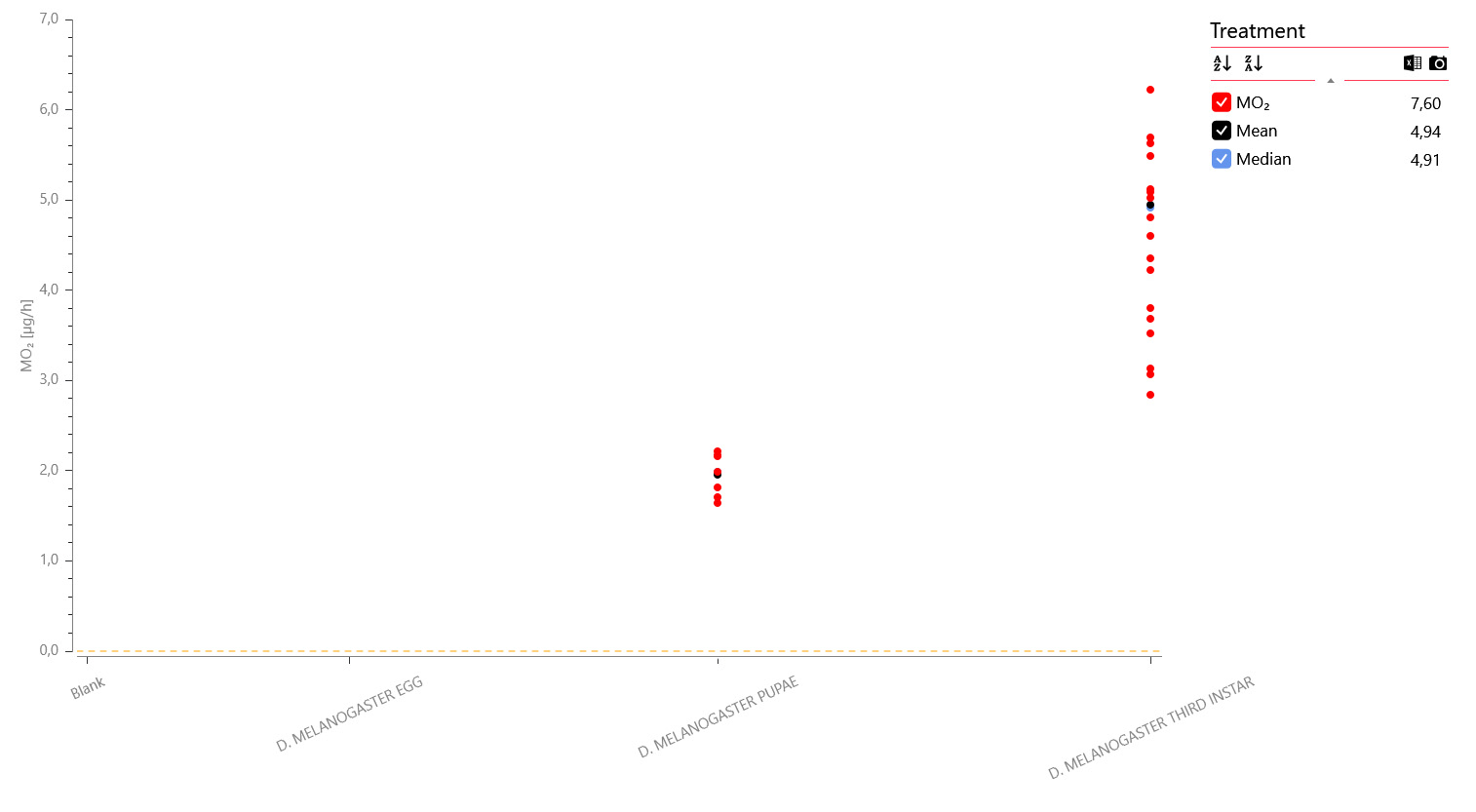
A data plot of the MO2 values shows oxygen consumption rates in the pupae (second from right) and larvae (first from right) but no consumption was detected in the eggs and blanks (far left):
The next part of the project investigated the respiration rates in adult fruit flies, here D. littoralis. Besides determining the oxygen consumption rates of adult individuals, we also wanted to look at the effects on MO2 from anesthetization with CO2 – a common anesthetic used when handling live fruit flies. An individual adult fruit fly was transferred using an aspirator tube from their maintenance chamber at 21 °C and then directly into a well or anesthetized for 30 sec. with CO2 prior to transfer. The 200 µl glass plate was then closed with sealing film and placed under a box.
The MicroResp™ v1.1 software was used to calibrate the oxygen sensors, set up the plate and experiment settings, and to log data. Here are the exported data graphs:
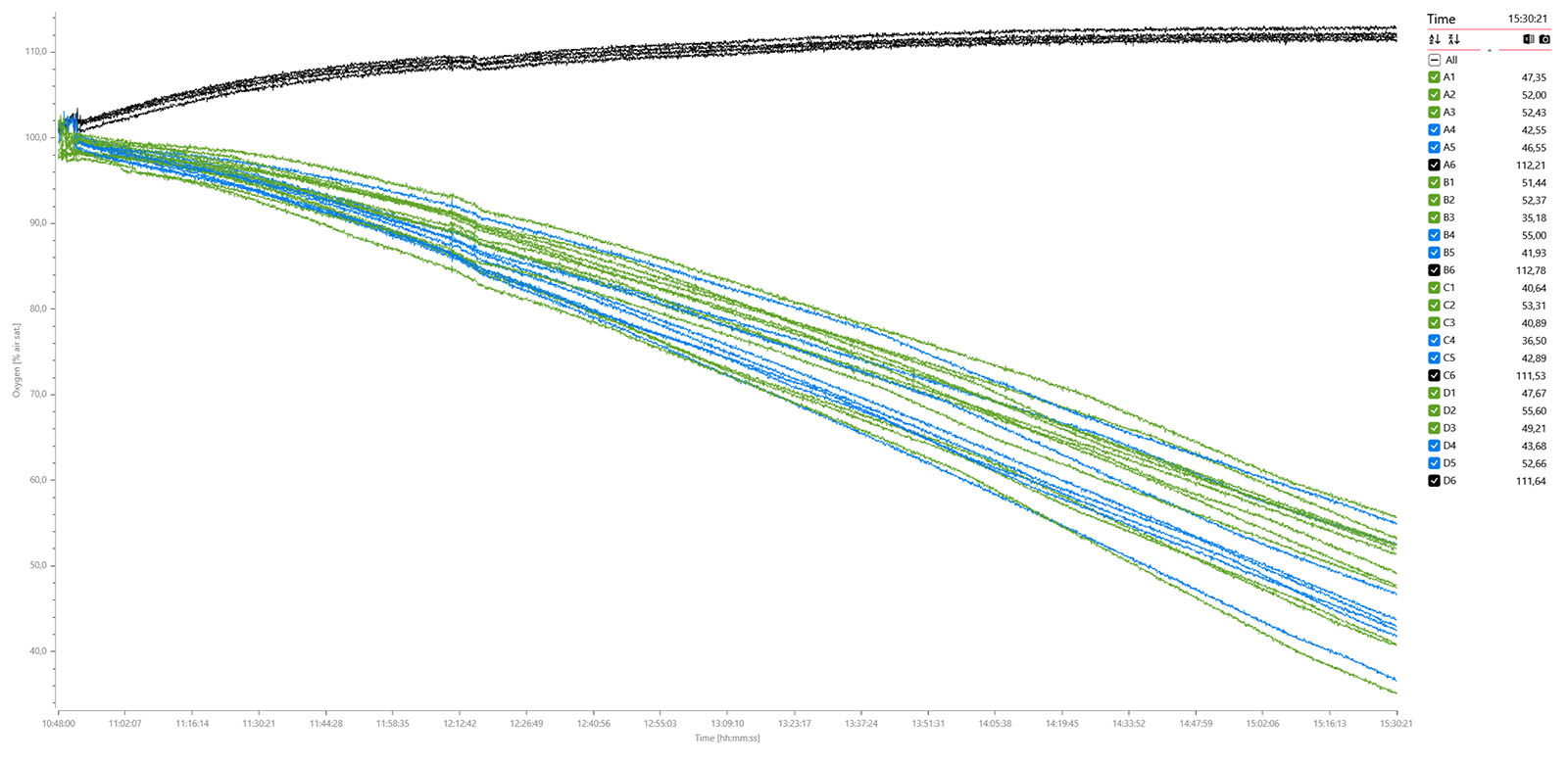
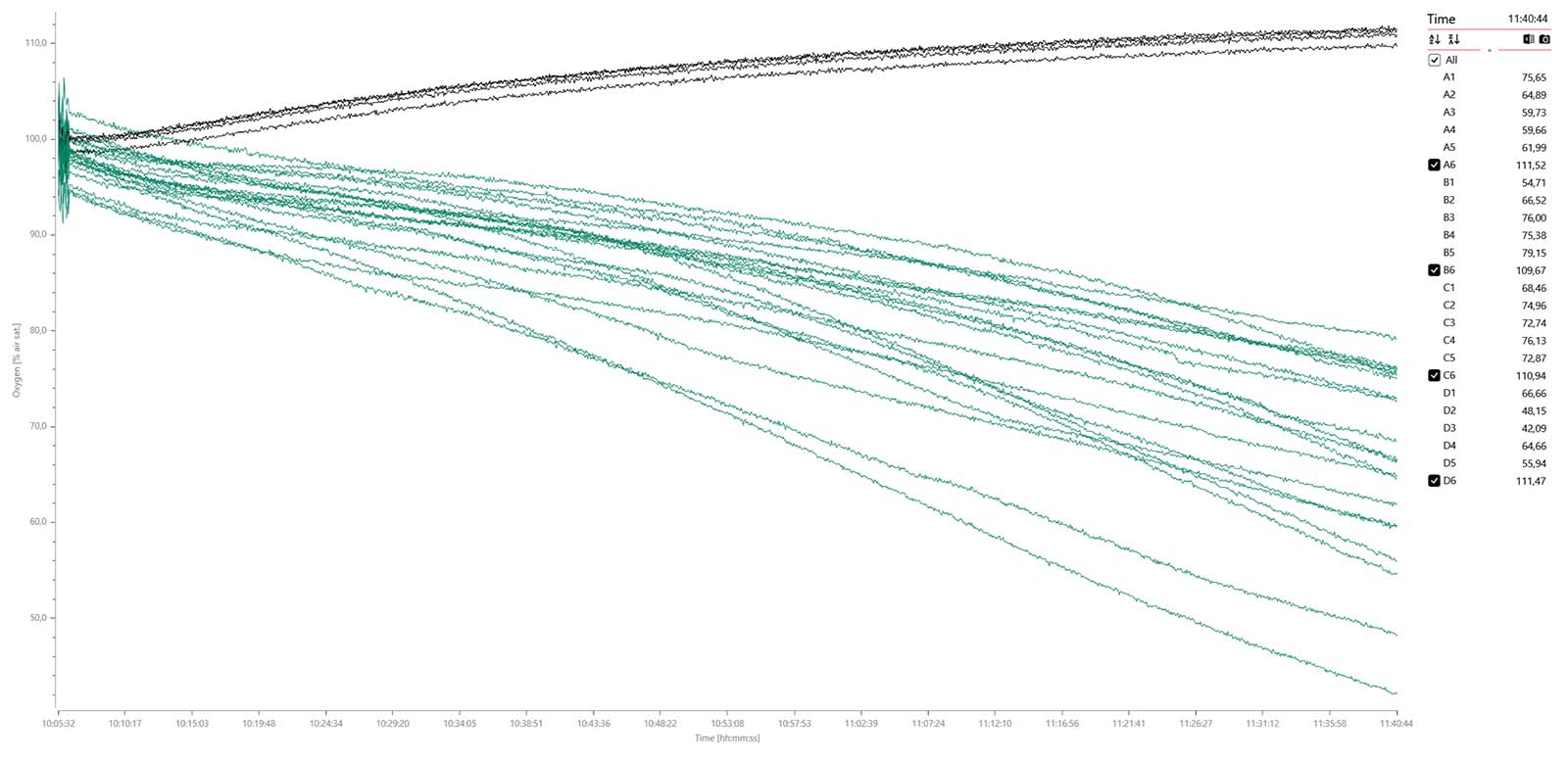
Oxygen data graph for 12 x adult flies (green), 8 x adult flies anesthetized with CO2 (blue), and 4 x blanks (black) measured over 4 hours and 41 min. A data plot of the MO2 values shows no apparent difference between non-anesthetized (mid) and anesthetized individuals (right):
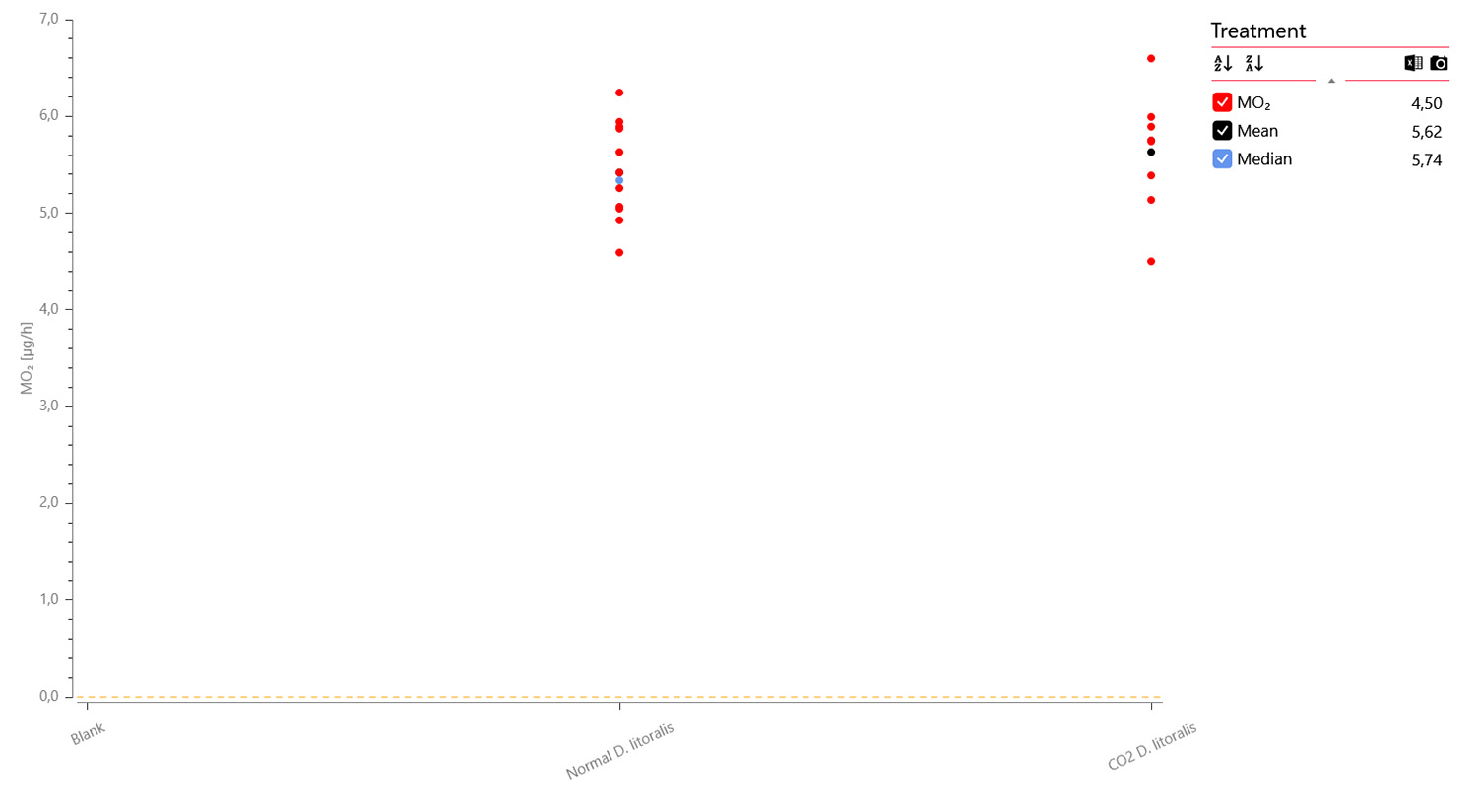
In conclusion, the microplate respirometry system was able to dertemine oxygen consumption rates in various life stages of fruit flies in a relative short period of time.

Want to learn more about the microplate respirometry system?