News
Sun, Jun 01 2025
Summer Holiday
Our office will be closed for the summer holiday from 12th - 27th of July (both days included).
We wish everyone a lovely summer!
The Loligo® team
Mon, Apr 28 2025
New high precision system for well plate filming

Enter the CentriTower system, a cutting-edge optical telecentric lens system designed to deliver high-resolution video images with zero parallax error and zero lens distortion. This innovative and compact system is perfectly suited for filming in PCR plates, deep well plates, petri dishes, and similar-sized vessels.

Researchers can now analyze the behavior of tiny, fast-moving organisms such as fish larvae, Daphnia, copepods, and Drosophila with exceptional clarity and precision. The system includes a 5 MP USB 3 camera with a C-mount, capable of capturing videos at up to 75 frames per second (FPS), ensuring that no detail is missed.

One of the standout features of the CentriTower system is its ability to block ambient lighting, thanks to its durable yet transportable tower design. This ensures consistent and reliable results by eliminating reflections and enhancing contrast. Additionally, the system includes an infra-red light panel for back illumination, which further improves video tracking performance without affecting the behavior of the test organisms.
The CentriTower system seamlessly integrates with our automated video tracking and behavior analysis software, LoliTrack v5 – a powerful combination of hardware and software allowing researchers to track and analyze behavior in small animals with ease.

As the scientific community continues to push the boundaries of knowledge, the CentriTower system stands out as a vital tool for researchers seeking to gain deeper insights into the intricate behaviors of the natural world. With its top-quality optical components and user-friendly design, the CentriTower system is set to become an indispensable asset in laboratories around the globe.
The CentriTower can be bought as a separate product or as a system that includes LoliTrack v5 and an IR light panel.
Wed, Apr 02 2025
Welcome to our new product specialist
We are happy to welcome and introduce our new product specialist, Louise Vinther Grøn, to the Loligo Systems team. We have said goodbye to Andreas Mørck, who after 8 years in this position is moving to a new position in a biotech company. We thank him for all his great work here at Loligo Systems and wish him all the best of luck in his new job!
Louise joins our team from Aarhus University, where just last week she successfully defended her PhD in Biological and Chemical Engineering. During her PhD studies, Louise worked with acetogenic microorganisms in bioelectrochemical systems. From this work, she has a strong background in the integration of measurement and sensor technologies into specialized systems to investigate biological processes, and before that, an educational background in Biology. Building on this experience, Louise will now provide our customers at Loligo Systems with valuable, efficient and free support on our systems – as we have always offered.
With her background in microbiological systems, Louise will also explore how our measurement systems can be applied for microbial oxygen consumption and respirometry. If you are curious about the possibilities of using our systems for your microbiological experiments, please reach out to Louise!
Fri, Nov 15 2024
New Job Opportunity
Product Specialist with a flair for technology and a passion for bio sciences
Do you want to work with leading technologies for measuring respiration and behavior in animal models such as Zebrafish, Daphnia, and other aquatic organisms? Do you have a background in biology, biotech, biochemistry, or environmental engineering, and are you passionate about measurement technology, technical support, and the development of advanced equipment? If so, the role of Product Specialist at Loligo® Systems could be your next career step.
About the Position
As a Product Specialist at Loligo® Systems, you will be a key figure in our company and have a unique opportunity to work with researchers and students from universities worldwide. Building on your academic background in biology, biochemistry, or related fields—ideally with a Ph.D. or equivalent—you will provide guidance on using our advanced measurement equipment, participate in testing, and developing existing and new products, and be responsible for documentation in the form of web content, technical drawings, illustrations, and more.
Your Primary Responsibilities:
- Technical support for customers via email and Teams/Zoom
- Development and updating of web content and product descriptions in our CMS system (Umbraco)
- Documentation and illustration of technical drawings and product functionalities
- Participation in international conferences and trade shows
- Contribution to product development, testing, and quality control
After thorough training on our systems and products, you will have the opportunity to shape the role based on your strengths and interests. You will report directly to the owner-manager and take on a central role in a small company, where you can influence development and become a key figure with management responsibility.
Who You Are:
- You have a relevant educational background, preferably with a Ph.D. in biology, biochemistry, biophysics, or related engineering fields, and a strong interest in measurement methods and innovation
- Experience from research environments or previous work with measurement equipment is an advantage, and you feel comfortable in a role with direct customer contact.
- You thrive in a varied workday, where you can focus on tasks related to technical documentation and quality assurance, while also providing efficient support to researchers globally via email and e-meetings
- You have strong language (English) skills, both written and verbal, and communicate clearly and precisely, whether with researchers, students, or professionals from diverse cultural backgrounds
- You are systematic and quality-oriented, with a natural curiosity about how technology can support research. Experience with CMS, CAD, or other digital systems is an advantage
We Offer
At Loligo® Systems, you’ll be part of a small, dynamic team with high professional standards, where innovation and technical development are the focus. As a Product Specialist, you’ll work in a specialized environment that provides solutions to a global customer base of researchers and universities who expect precision, deep insight, and high quality in all technical support. Our clients are knowledge-intensive experts in their fields, and they greatly value the high standard and technical consultation we offer.
At Loligo® Systems, you have the chance to quickly take on responsibility and make a noticeable difference in developing groundbreaking solutions for the research community. We work in modern facilities in Viborg—just a two-minute walk from the train station—with a canteen and health schemes that support a positive work environment. We also offer an attractive compensation package with health insurance and pension benefits, and we place a high priority on individual professional development and engagement.
Application
Career | a JKS company is handling the recruitment process. If this position sounds like the perfect match for you, we would love to hear from you! Please send your CV and a brief, motivated application with relevant attachments via the "Apply now" button below. We review applications on an ongoing basis, so feel free to apply today.
If you have any questions that are not covered in the job listing, please feel free to contact Chief Consultant Anne-Marie Hviid Ekstrøm at +45 3085 1294.
Please note that applications sent directly via email will not be processed or answered due to GDPR regulations.
Link to application: https://tinyurl.com/34cbhssc
LinkedIn post: https://tinyurl.com/5anhk4s7
Facebook post: https://fb.watch/vREf4iaTh6/
Thu, Jun 13 2024
How to measure respiration rates in Drosophila
In this article, we showcase how the microplate respirometry system provides an easy to use, high throughput respirometry tool for determining oxygen consumption rates in various life stages of terrestrial insects, here using fruit flies (Drosophila melanogaster and Drosophila littoralis). The project was performed at Aarhus University in Denmark in collaboration with Prof Jesper G. Sorensen at Aarhus University in Denmark.
The first part of the project looked at the respiration rates in eggs, third instar larvae, and pupae of D. melanogaster. The fruit flies were maintained at 19 °C, and were in the following life stages:
- melanogaster eggs: 0-12 hours old
- melanogaster larvae: 6-7 days since egg stage
- melanogaster pupae: 8-9 days since egg stage
The first trial had 10 x eggs + 10 x pupae (+ 4 x blanks). The eggs and pupae were transferred directly from their food solution/colony and placed individually in a well in the 80 µl glass microplate. The wells were then closed using gas-tight non-toxic sealing film, and placed onto the microplate reader. The microplate reader and glass plate were then covered with an opaque plastic box, to prevent ambient light from interfering with the optical oxygen sensor inside each well, and left at room temperature (21 °C). Here is a picture of the setup:

The MicroResp™ v1.1 software was used to calibrate the oxygen sensors, set up the plate and experiment settings, and to log data. Here are the exported data graphs:
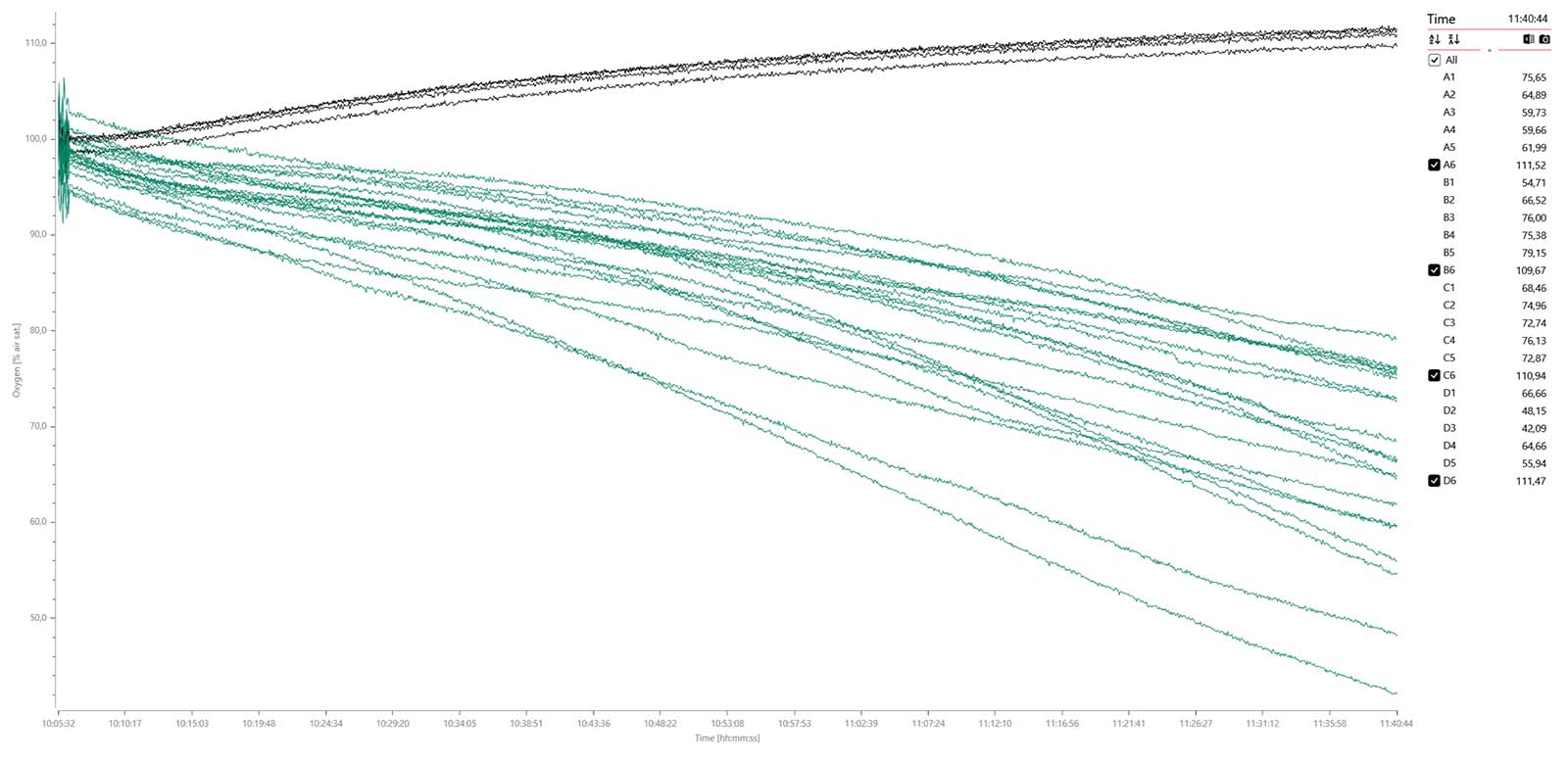
Oxygen data graph for 10 x eggs (dark golden), 10 x pupae (purple), and 4 x blanks (black) measured over 2 hours and 18 min.
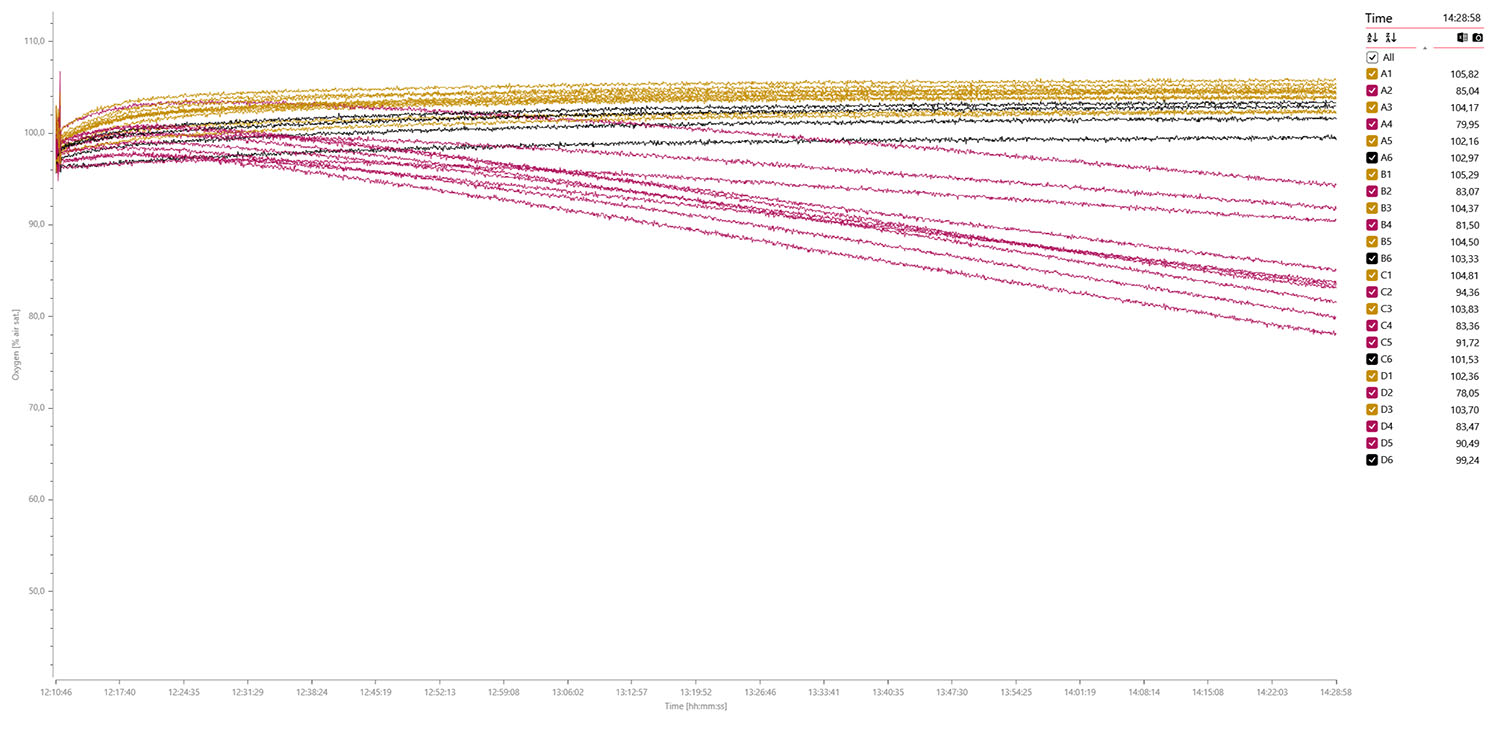
The next trial used 20 x third instar larvae (dark cyan) and 4 x blanks (black), and data was logged for 1 hour and 35 min.:
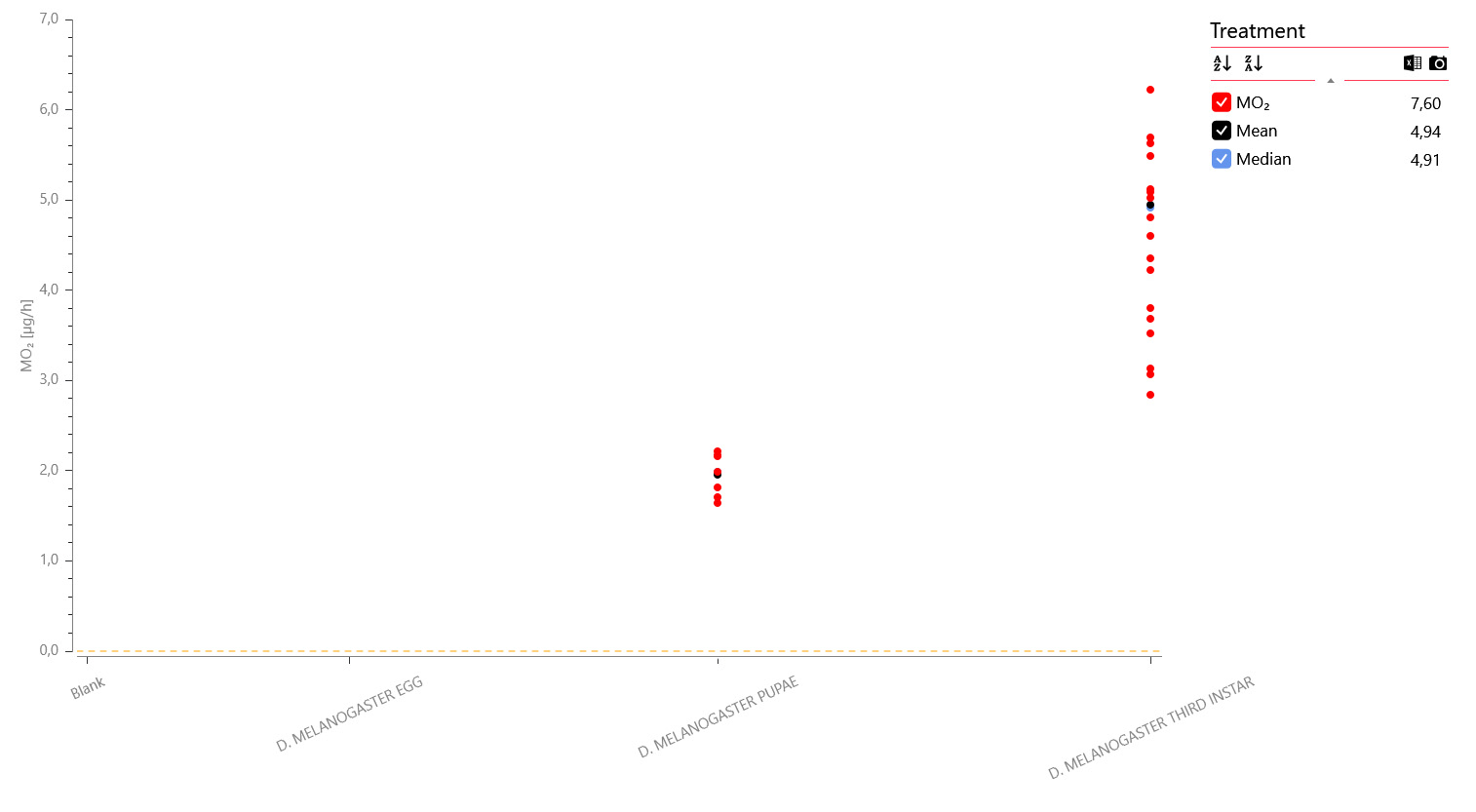
A data plot of the MO2 values shows oxygen consumption rates in the pupae (second from right) and larvae (first from right) but no consumption was detected in the eggs and blanks (far left):
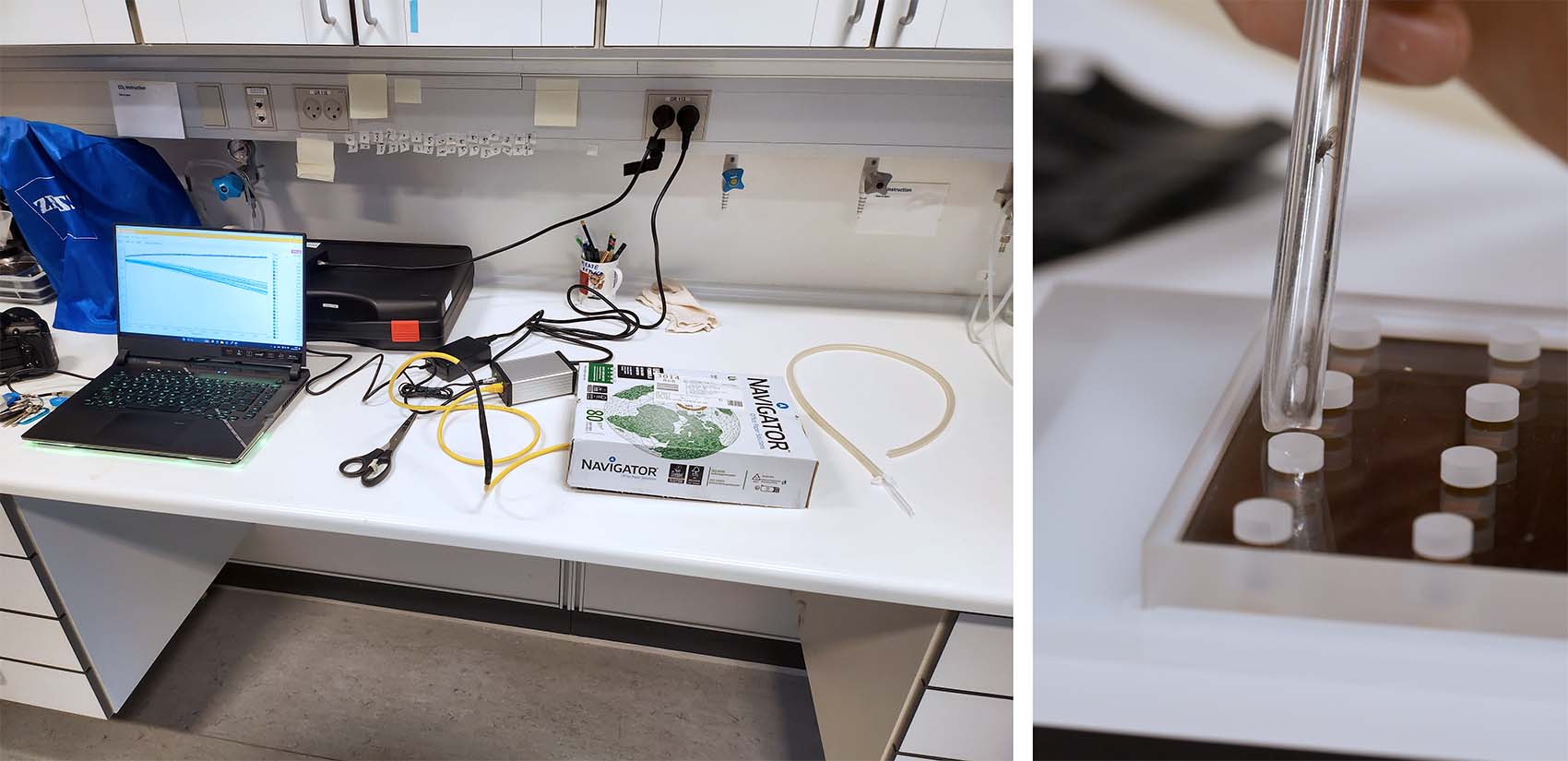
The next part of the project investigated the respiration rates in adult fruit flies, here D. littoralis. Besides determining the oxygen consumption rates of adult individuals, we also wanted to look at the effects on MO2 from anesthetization with CO2 – a common anesthetic used when handling live fruit flies. An individual adult fruit fly was transferred using an aspirator tube from their maintenance chamber at 21 °C and then directly into a well or anesthetized for 30 sec. with CO2 prior to transfer. The 200 µl glass plate was then closed with sealing film and placed under a box.
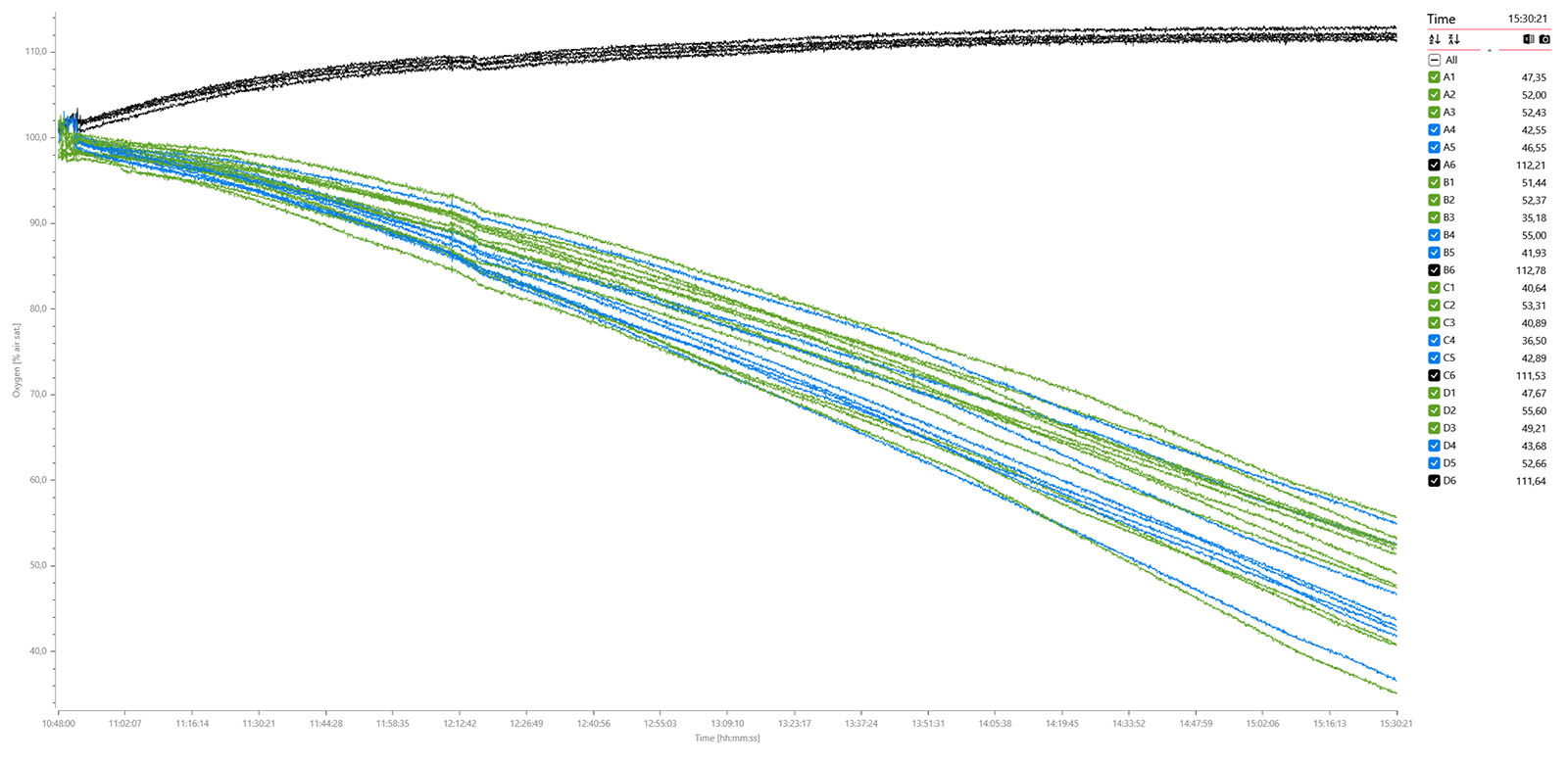
The MicroResp™ v1.1 software was used to calibrate the oxygen sensors, set up the plate and experiment settings, and to log data. Here are the exported data graphs:
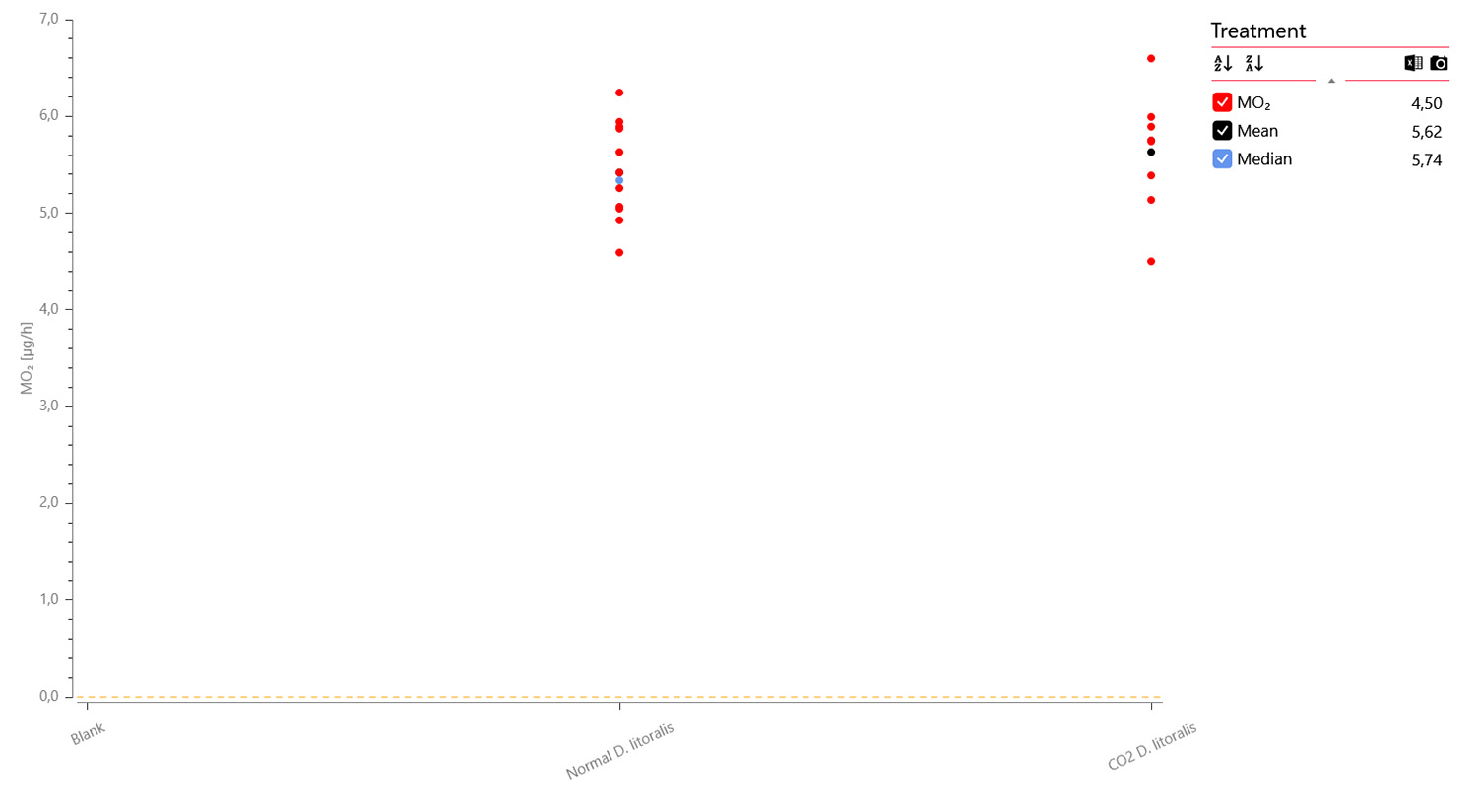
Oxygen data graph for 12 x adult flies (green), 8 x adult flies anesthetized with CO2 (blue), and 4 x blanks (black) measured over 4 hours and 41 min. A data plot of the MO2 values shows no apparent difference between non-anesthetized (mid) and anesthetized individuals (right):
In conclusion, the microplate respirometry system was able to dertemine oxygen consumption rates in various life stages of fruit flies in a relative short period of time.

Want to learn more about the microplate respirometry system?
Wed, May 29 2024
Introducing the new LoliGO App
We are excited to offer users of Loligo® software this new free and useful tool.
The LoliGO App is a smartphone application for Android and iOS that allows users to remotely monitor their experiments while having the option of getting push notifications in case of power outs in the lab, computer failures, or alarms based on water quality thresholds – like if the temperature gets critically high in your respirometry chambers.



The LoliGO App uses a secure cloud-based service to handle data sent from the Loligo® software and to the App. Users can set up a user profile, and multiple users can access the same user profile at the same time (if they know the password!) aka you can have a shared lab profile.
You cannot alter or stop experiments from the app, and you cannot export or otherwise gain access to the raw data from the app. Completed experiment data will be automatically deleted from the app after 14 days.
The LoliGO App is free to use, and is currently compatible with AutoResp™ v3, and the app will be available with future updates of other Loligo® software soon.
Download the latest version of the LoliGO App:
- LoliGO for Android (on Google Play)
- LoliGO for iOS (on App Store)
Mon, Apr 29 2024
Sponsorship 2024
Once again, we are honored to be supporting the scientific community. In 2024, we are sponsoring a list of symposia, workshops, and meetings, and we encourage all interested to take a closer look at the following events:
- We have sponsored travel money for invited speakers at the very interesting SEB 2024 Symposium ”Balancing energy acquisition, expenditure, and allocation in an ever-changing world” through Dr. Amanda Pettersen (co-chairs: Neal Dawson, Ed Ivimey-Cook), and we hope to see you all for a great event in Prague, July 2-5!
- We have donated cash to support students going to the CSZ-SCZ annual meeting on May 6-10, 2024, in Moncton, NB.
CSZ-SCZ 2024 - Home / Accueil (cszsczmoncton2024.com) - Once again, we have accepted to be ICBF 2024 Student Travel Sponsors to help young people participate in this biennial meeting.
International Congress on the Biology of Fish 2024 | 15th (michiganseagrant.org)
We also support this meeting through a Lake Superior Sponsorship and participate as exhibitors as we did for nine (9) consecutive ICBF meetings
See you in Ann Arbor Jun 23-27! - We are proud sponsors of the 47th annual Larval Fish Conference in Huron, Ohio, on May 12-16, and hope that it will be a great event.
47th Larval Fish Conference - We are also proud sponsors of the upcoming Midwest Zebrafish Conference 2024 taking place June 7-9 at Washington University in St. Louis.
Check out the meeting right here: Midwest Zebrafish Meeting 2024 - We give financial support to a workshop on metabolism in animal ecophysiology arranged by Dr. Christel LeFrancois, Dr. Marie Vagner (Brest) and Dr. Loïc Teulier (Lyon). This seminar will take place on October 2-3, 2024, in La Rochelle, France.
For more details, please visit: https://www.rt-metabolica.cnrs.fr/
Thu, Apr 04 2024
Fish resting respirometry 101
Image: Male toadfish showing strong paternal care for 10 DPF brood. Image by Martin Grosell, RSMAS, University of Miami.
If you are looking for a solid fish respirometry and hypoxia paper with thorough explanations on methods, protocols, and data analysis, look no further than this 2023 publication by LeeAnn Frank, Joseph Serafy, and Prof. Martin Grosell from the Rosenstiel School of Marine, Atmospheric, and Earth Science, University of Miami.
A large aerobic scope and complex regulatory abilities confer hypoxia tolerance in larval toadfish, Opsanus beta, across a wide thermal range
Frank, LeeAnn; Serafy, Joseph; Grosell, Martin (2023)
Science of The Total Environment
The team of researchers provides an easy-to-follow methodical description on how to measure standard metabolic rate (SMR), routine metabolic rate (RMR), maximum metabolic rate (MMR), and aerobic scope (absolute and factorial) as well as how to assess the regulation index, P50, and Excessive Post Hypoxia Consumption (EPHOC) data in larval toadfish (Opsanus beta) over a range of their environmental temperatures. In addition to the detailed materials and method section, the authors supply the article with a manageable overview on how to analyze such respirometry data and which data parameters to include. The data are effectively illustrated using an assortment of data graphs, plots, and tables.
If you have any questions about the paper, you are welcome to contact LeeAnn Frank at leeann.frank@earth.miami.edu.
Want to know more about the respirometry system used in this paper?
Tue, Jan 23 2024
USER CASE – Testing microplate system against traditional gas analysis
In a peer-reviewed publication by Earls et al. (2023), a team of researchers from North Dakota State University and USDA compares the Loligo® microplate respirometry system with a traditional gas analyzer setup in a study measuring the metabolism of using alfalfa leafcutting bees (Megachile rotundata) and effects of temperature. The paper can be found here:
Earls, Kayla N; Campbell, Jacob B; Rinehart, Joseph P; Greenlee, Kendra J
- Biol Open (2023) 12 (12): bio060213.
- https://doi.org/10.1242/bio.060213

Daisy-chained microplate reader with glass plate on top.
The Loligo® microplate respirometry system used in this study featured two daisy-chained microplate readers using glass plates with 940 µl wells for simultaneous measurements of up to 48 bees at a time. Both readers were controlled using our MicroResp™ software, which was also used for calculating the V̇O2 slope and to randomly assign bees to the different wells.

A mix of brood cell and pupae M. rotundata in 940 µl wells. Photo by Kayla Earls.
The 48-chamber microplate setup was compared to a traditional closed respirometry setup with manual injections of air samples into a CO2 analyzer (Li-Cor-7000, LI-COR Biosciences, Lincoln, NE, USA) and O2 (Oxzilla FC-2 Differential Oxygen Analyzer, Sable Systems International, Las Vegas, NV, USA) gas analyzer.
The scientists found no significant differences between the V̇O2 data from the two different setups when comparing mass-specific metabolic rate calculations:

Copied from Fig. 1. in the publication.
But the setup comparison shows a significant difference in the time (and resources) spent on collecting the data:
“Pupal bees in the traditional closed respirometry system remained in the [individual] syringes for longer periods (∼9 h) than bees tested in the microplate system (∼3 h) due to the differences in chamber size (3 ml syringes versus 940 µl wells) and detection limits of the oxygen analyzers.”
It is also worth mentioning that a microplate system can analyze up to 240 organisms at a time, while the bees in the traditional closed respirometry had to be analyzed one at a time. In other words, the time spent on collecting data is substantially shorter in the microplate system.
Most impressively, the researchers present a vast dataset on oxygen consumption rates in the leafcutting bees across a large range of temperatures (6 – 48 °C) and developmental stages and between sexes:

Copied from Fig. 2. in the publication.

Copied from Fig. 4. in the publication.
If you are interested in learning more about this project or its team, you are welcome to contact Kayla N. Earls (Kayla.Earls@wsu.edu).
Want to learn more about the microplate respirometry system?
Tue, Dec 12 2023
New MicroResp™ version 1.1.0
We have updated the MicroResp™ application to version 1.1.0. You can download this latest version here:
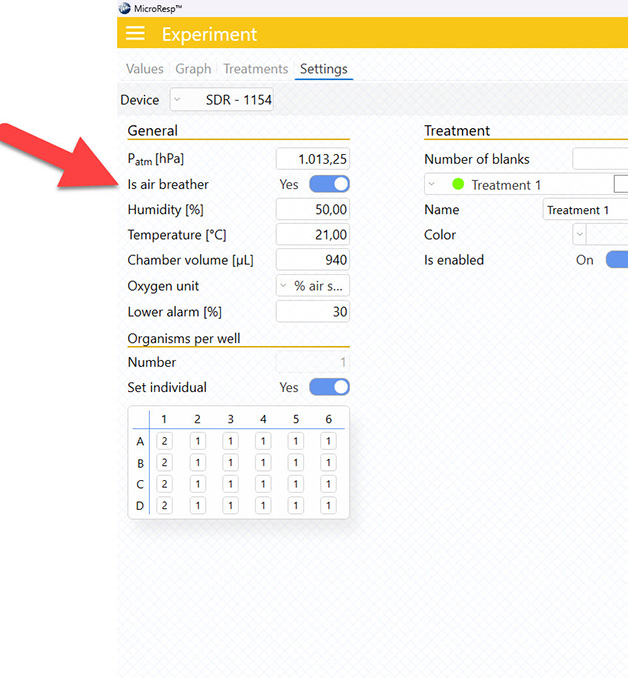
MicroResp™ 1.1.0 introduces some exciting new features including MO2 measurements in in air/gas for scientists working with air-breathers like adult insects (e.g., Drosophila, mosquitoes, solitary bees, midges, etc.) or terrestrial invertebrates (e.g., amphipods, isopods, etc.). Simply enable this feature in the Settings tab, while running an experiment, and enter the relative humidity below.
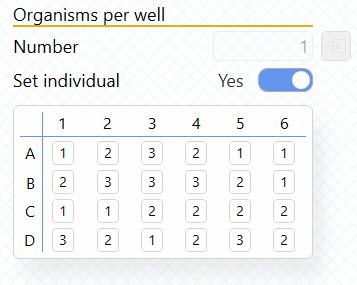
Another requested feature is that you can now specify the number of organisms per well. Previously, MicroResp™ assumed that you had the same number of organisms in each well, but you can now set the individual number for each of the 24 wells, and get MO2 values per organism. Enable “Set individual” under Organisms per well in the Settings tab, and then type in the number of organisms for each well.
Finally, we have added a timestamp to each MO2 value in the data file.