FAQ
What is intermittent respirometry?
The following references offer a good start to understanding the principles, methods, and what to look out for when studying intermittent respirometry in aquatic organisms:
- A large aerobic scope and complex regulatory abilities confer hypoxia tolerance in larval toadfish, Opsanus beta, across a wide thermal range
LeeAnn Frank, Joseph Serafy, Martin Grosell (2023)
Science of the Total Environment
Link to article - Methods for Fish Biology, 2nd edition
Timothy D. Clark (2022)
Respirometry. Pages 247–274 in S. Midway, C. Hasler, and P. Chakrabarty, editors. Methods for fish biology, 2nd edition. American Fisheries Society, Bethesda, Maryland.
Link to article - Guidelines for reporting methods to estimate metabolic rates by aquatic intermittent-flow respirometry
Shaun S. Killen, Emil A. F. Christensen, Daphne Cortese, Libor Závorka, Tommy Norin, Lucy Cotgrove, Amélie Crespel, Amelia Munson, Julie J. H. Nati, Magdalene Papatheodoulou, David J. McKenzie (2021)
Journal of Experimental Biology
Link to article - Effects of social and visual contact on the oxygen consumption of juvenile sea bass measured by computerized intermittent respirometry
J. Herskin (2005)
Journal of Fish Biology
Link to article - Some errors in respirometry of aquatic breathers: How to avoid and correct for them
J. F. Steffensen (1989)
Fish Physiology and Biochemistry
Link to article
Intermittent flow (or stop-flow) respirometry - Principles and Background
Measurements of oxygen consumption rates on fish and other water breathers commonly involves using one of three different methods:
1. Closed respirometry (or constant volume respirometry)
2. Flow-through respirometry (or open respirometry)
3. Intermittent flow respirometry (or stop-flow)
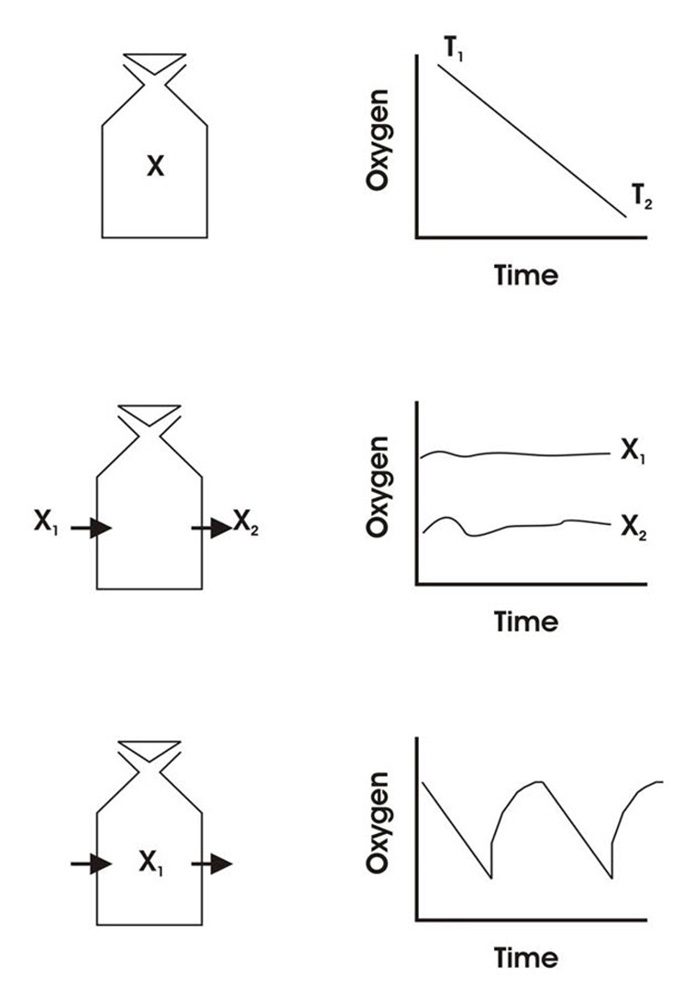
1. Closed respirometry (or constant volume respirometry)
Measurements in a sealed chamber of known volume (a closed respirometer). The oxygen content of the water is measured initially (t0), then the respirometer is closed and at the end of the experiment (t1) the oxygen content is measured again.
Knowing the body weight of the animal, the respirometer volume and the oxygen content of the water at time t0 and t1, the mass specific oxygen consumption rate (mg O2/kg/hour) can be calculated as follows:
VO2 = ([O2]t0 – [O2]t1) • V/t/BW
- [O2]t0 = oxygen concentration at time t0 (mg O2/liter)
- [O2]t1 = oxygen concentration at time t1 (mg O2/liter)
- V = respirometer volume minus volume of experimental animal (liter)
- t = t1 – t0 (hour)
- BW = body weight of experimental animal (kg)
An advantage of this method is its simplicity. A disadvantage is that the measurements are never made at a constant oxygen level, due to the continuous use of oxygen by the animal inside the respirometer. This might cause problems when interpreting data, since animal respiration often changes with ambient oxygen partial pressure.
Furthermore, metabolites from the experimental animal, i.e. CO2, accumulate in the water, thus limiting the duration of measurements. This limited time for measurements prevents the experimental animal to recover from initial handling stress that often increase fish respiration significantly and for several hours, thus overestimating oxygen consumption rates.
2. Flow-through respirometry (or open respirometry)
This is a more sophisticated method for oxygen consumption measurements. Experimental animals are placed in a flow-through chamber with known flow rate. Oxygen is measured in the inflow and outflow and oxygen consumption rate (mg O2/kg/hour) can be calculated as:
VO2 = F • ([O2]in – [O2]out)/BW
- F = water flow rate (l/hour)
- [O2]in = oxygen content in water inflow (mg O2/liter)
- [O2]out = oxygen content in water outflow (mg O2/liter)
- BW = body weight of experimental animal (kg)
The advantages of this method are several:
- The duration of the experiment is in principle unlimited
- No accumulation of CO2 and other metabolites
- Its possible to measure at a constant oxygen level
- By controlling the quality of the inflowing water its possible to measure metabolism at different desired levels of oxygen, salinity etc.
However, this method bring along one significant disadvantage: In order to determine oxygen consumption by open respirometry it is crucial that the system is in steady state. This means that the oxygen content of the in-flowing and out-flowing water, AND the oxygen consumption of the animal, have to be constant.
If the oxygen consumption of the animal for some reason changes during the experiment, steady state will not exist for a while. The above formula will not give the correct oxygen consumption rate until the system is in steady state again. The duration of the time lag depends on the relationship between chamber volume and flow rate. Thus, open respirometry measurements have poor time resolution and are not suitable for determination of oxygen consumption on organisms with a highly variable respiration like fish.
3. Intermittent flow respirometry
Our system for automatic respirometry works by intermittent flow respirometry aiming at combining the best of both 1) closed and 2) flow-through respirometry.
- The experimental animal is placed in a respirometer immersed in an ambient tank (temperature bath).
- A recirculating pump ensures proper mixing of the water inside the respirometer and adequate flow past the oxygen probe.
- A second pump can swap the water inside the respirometer with water from the ambient tank (temperature bath). During measurements of oxygen consumption, this flush pump is turned off and the systems operates like a closed respirometry setup.
- Then the pc controlled flush pump turns on pumping ambient water into the respirometer and bringing the oxygen content back to pre measurement levels.
In this way, problems with accumulating metabolites and severe changes in oxygen level due to animal respiration are avoided. - As with open respirometry, the duration of the experiment is in principle unlimited.
However, the most important advantage is the great time resolution of this method. Oxygen consumption rates of animals can be determined for every 10th minutes over periods of hours or days, making our systems for automatic respirometry extremely suited for uncovering short term variations (minutes) in respiration.
In summary, our system for automatic respirometry is developed for prolonged and automatic measurements of oxygen consumption rate in a controlled laboratory environment.
The automatic measuring proceedure runs in 3 phases:
- Measuring period
- Flush period
- Wait period
In the Measuring period (1) the flush pump is off, and the chamber is closed. Fish respiration rate is calculated from the decline in oxygen. During this time the recirculation pump is active to mix the water inside the respirometer and to ensure proper flow past the oxygen sensor.
The measuring period is followed by a Flush period (2) where the flush pump is active pumping water from the ambient temperature bath and into the respirometer. During this period the recirculation pump is inactive and the oxygen curve will raise to approach the level of the amient water.
Finally, the flush pump stops and the loop ends with a short Wait period (3) before starting a new measuring period. This waiting period is necessary to account for a lag in the system response resulting in a non-linear oxygen curve. During the Wait period the recirculation pump is active.
Examples of raw MO2 data
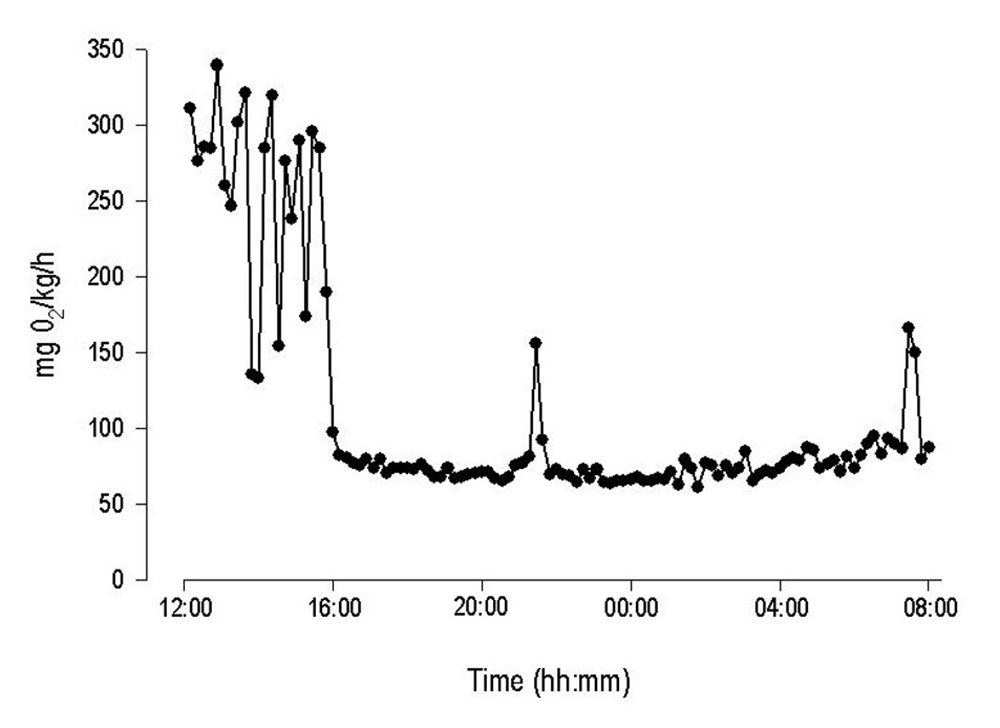
Standard metabolic rate of juvenile Rainbow Trout was determined in static respirometer chambers and with an automated respirometry system from Loligo® Systems. Initial high oxygen consumption rates due to handling stress, were followed by a gradual decline to lower and more stable values indicating standard metabolic rate for the specimen. Notice the high temporal resolution (10 min) of the system revealing sudden changes in MO2.
Courtesy by J.Svendsen/DIFRES & J.Lund/Univ. Aarhus
LinkWhat chamber sizes will fit my animal?
The chamber should be large enough to house the intact animal, but not much larger as it will leave space for unwanted activity like swimming that will make it harder (and take longer) to determine resting/standard/minimum metabolic rate.
The chamber volume should be kept at a minimum for reliable respiration measurements to get a better temporal resolution and signal-to-noise ratio. If the volume is too large, the resulting oxygen depletion curve will be too flat for solid estimates of the slope that is used in the calculation of the oxygen consumption rate (MO2).
As a rule of thumb, we recommend a resting chamber with a volume up to 50 times the volume (≈ wet weight) of the animal:
Use a 500 mL chamber for fish weighing down to 10 g (ratio = 500/10 = 50).
A lower ratio might be needed as the mass specific respiration rate of ectotherm animals, like fish, scales with body size (i.e., large animals have lower mass specific O2 consumption rates than smaller animals) and temperature (i.e., high temperature = high respiration and vice versa), and also differs between species (e.g., a sluggish benthic fish species often have a lower mass specific metabolic rate than an active pelagic species).
Thus, use a smaller chamber (ratio) if working with large and sluggish arctic fish, e.g., a 15 L chamber for fish weighing down to 1 kg fish (ratio = 15/1 = 15).
LinkWhat swim tunnel should I choose?
Active fish swimming in a tunnel respirometer have high mass-specific oxygen consumption rates (MO2), allowing reliable oxygen consumption estimates in relative higher respirometer volume than for static respirometry. Tunnel respirometers are difficult to build with a fish volume to respirometer volume of less than 1:100. We recommend a maximum ratio between the wet weigth (or volume) of the fish and the volume of the swim respirometer of 1:200, e.g. a 90 liter swim respirometer fits fish down to app. ½ kg. If the volume is too large, the resulting oxygen curve will be too flat for reliable estimates of the slope that is used in the calculation of oxygen consumption rate (MO2).
Another restraint is the dimensions of the test section, which should allow the experimental fish to perform unrestricted swimming. This will largely depend on the species and mode of swimming.
Please contact us to get free advice on which swim tunnel that will be best for your project.
LinkWhat pump flow is needed for my chamber?
To wash out a tube-shaped respirometer chamber requires a volume ~5 times that of the chamber.
Thus, if you use a pump delivering a flow per minute equal to the chamber volume, then a 5 minutes flush period will ensure that 99 % of the water is replaced during each flushing cycle.
Stronger pumps might create too strong a flow forcing fish to struggle or unwanted activity. Lower flows require longer time for flushing, and this will decrease time resolution of MO2 data.
LinkAre glass chambers better than plastic chambers?
Some materials, like Plexiglass/Perspex can act as oxygen stores and sinks, forming pools of oxygen which can be released or stored in a reversible way - see Stevens, J. (1992) J. Appl. Physiol. 72, 801-804.
This can have an important effect when chambers are down-scaled for micro respirometric measurements of very low oxygen fluxes.
Thus, for oxygen measurements in small volumes less than a few millilitres (mL), we recommend chambers of glas and inert components with non or low oxygen storage capacity!
For respirometers with larger volumes (>½ litre), the use of acrylic materials have no significant effects on measurements.
Link